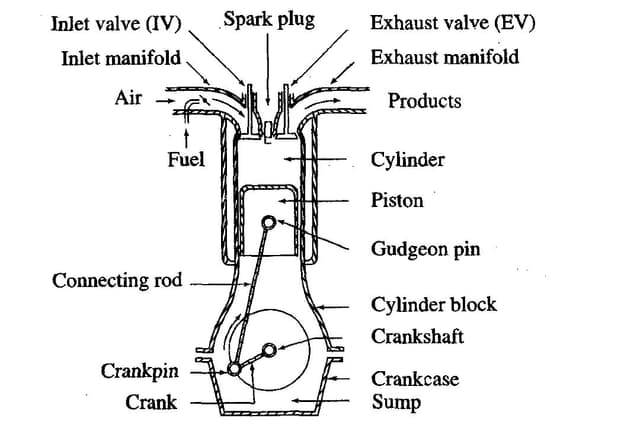
Sulphation
What is Sulphation

Bablu Yadav
Posted in Automobile Engineering
.
Source: UniEnergy Technologies Battery
What is Sulphation
When a battery is used, small lead sulphate crystals are formed. This is normal and does not harm the battery.
When a battery is deeply discharged a soft layer of sulphate is deposited on the electrodes. This will inhibit the performance of the battery. It can be reversed by overcharging.
If the battery stays in a low state of charge for longer periods, weeks or months, the soft layer of sulphate will convert into hard crystalline and become permanent. The battery will lose its capacity and eventually become useless.
To prevent permanent sulphation, top charge the batteries every three weeks. If they have been heavily drained, try to recharge as soon as possible.